
By Brad Hayes
Many people see hydrogen as a foundation of the zero emissions energy sources envisioned for later this century.
Hydrogen burns to create energy, producing only water vapour as a combustion product. So – no carbon dioxide, the greenhouse gas produced by burning oil and gas, which so many worry about causing climate change.
Actually, water vapour is a greenhouse gas, too, but there is so much of it in the atmosphere already that burning hydrogen would make no measurable difference.
Hydrogen has been proposed as a household fuel – either on its own or blended with natural gas for heating, water heating, and cooking. Vehicles powered by hydrogen fuel cells are seen by some as preferable over battery electric vehicles – particularly heavy trucks that would need massive batteries. And hydrogen stored in underground reservoirs could act as low-emissions backup for electrical grids when other low-emissions fuels are not available.
Hydrogen is potentially so useful and versatile that many are counting on it to be a foundational energy source:
- The Government of Canada’s hydrogen strategy aims to have 30% of end-use energy to be from clean hydrogen by 2050 (Hydrogen strategy for Canada)
- The United States just released its National Clean Hydrogen Strategy and Roadmap (U.S. National Clean Hydrogen Strategy and Roadmap)
- The United Kingdom Hydrogen Strategy is striving for 5GW of low-carbon hydrogen production capacity by 2030 (U.K. hydrogen strategy)
In fact, do a Google search of “hydrogen strategy” for just about any country, state, or province, and you will find detailed notes on how hydrogen will be produced and consumed within the next few years.
But not everyone is a hydrogen fan. It takes a lot of energy to manufacture hydrogen, regardless of the method. It is not an energy source; it is actually an energy vector – it transfers energy from one place to somewhere else where it can be useful, just as electricity moves from the power plant to our homes.
Hydrogen is relatively difficult to compress and to transport, and it is not very energy dense. Hydrogen leaking from pipes and facilities is thought to combine with other gases in the atmosphere, exacerbating the greenhouse warming effect. Some people call hydrogen “hopium”; they believe hydrogen promoters are way too optimistic about the role hydrogen has to play in our energy futures.
Up until now, the holy grail of hydrogen production has been “green” hydrogen – created by electrolyzing (chemically splitting) water using low-emissions energy sources, such as electricity from wind or solar. At the other end of the emissions spectrum, “grey” hydrogen is created from natural gas with carbon dioxide as a byproduct, and so is not a low-emissions fuel. In places such as Alberta, where we already produce a lot of grey hydrogen for industrial purposes, we are capturing and sequestering (storing underground) the CO2, thus creating a product called “blue” hydrogen, considered to be a low-emissions fuel.
Each method to manufacture hydrogen has issues. Electrolysis generally requires ultra-pure water, and in such large quantities that it would be hard to source in many areas. Carbon capture and storage to support blue hydrogen requires energy itself, and does not capture all the emissions generated throughout the process.
Can “natural” hydrogen save the day?
Some hydrogen promoters are excited about finding and producing naturally occurring hydrogen from within the earth, in much the same way that we produce natural gas – by drilling wells and collecting the gas that flows out. That would make hydrogen a true low-emissions energy source, not just an energy carrier, because we would not have to manufacture it.
Is this potential game-changer, which some call “gold” hydrogen, really on the horizon?
Natural Hydrogen Ventures (Natural hydrogen is a cheap, always on and zero emission energy) thinks so. Referencing a 2020 paper (The occurrence and geoscience of natural hydrogen: a comprehensive view), they show more than 100 locations around the world where naturally occurring hydrogen has been detected, many as surface seeps or in salt mines (Figure 1).
They enthusiastically promote the merits of natural hydrogen, claiming:
- As cheap as fossil fuels with a cost of ~$1/kg
- 2-5x cheaper than other types of hydrogen
- Immune to volatile energy prices since it has no input costs
- “Always on” so that it can stabilize the energy grid
- Estimated to be able to meet global energy demand for thousands of years
Let’s think about these claims by applying knowledge and experience from an industry we know very well – drilling for and producing natural gas. Explorers have already applied natural gas exploration and development methods to the burgeoning helium industry, so why not for hydrogen? First, just detecting hydrogen (a hydrogen “show”) does not mean much. Every analysis of gas from the subsurface exhibits a variety of components, and examining old gas analyses for unusual helium concentrations is one way we prospect for helium. Finding hydrogen in small amounts is actually quite common; Figure 1 should have a lot more hydrogen shows from oil and gas wells in Western Canada, but whoever created the map did not examine our gas analysis databases.
Figure 1: Regions where naturally occurring hydrogen has been found. Source: Natural Hydrogen Ventures
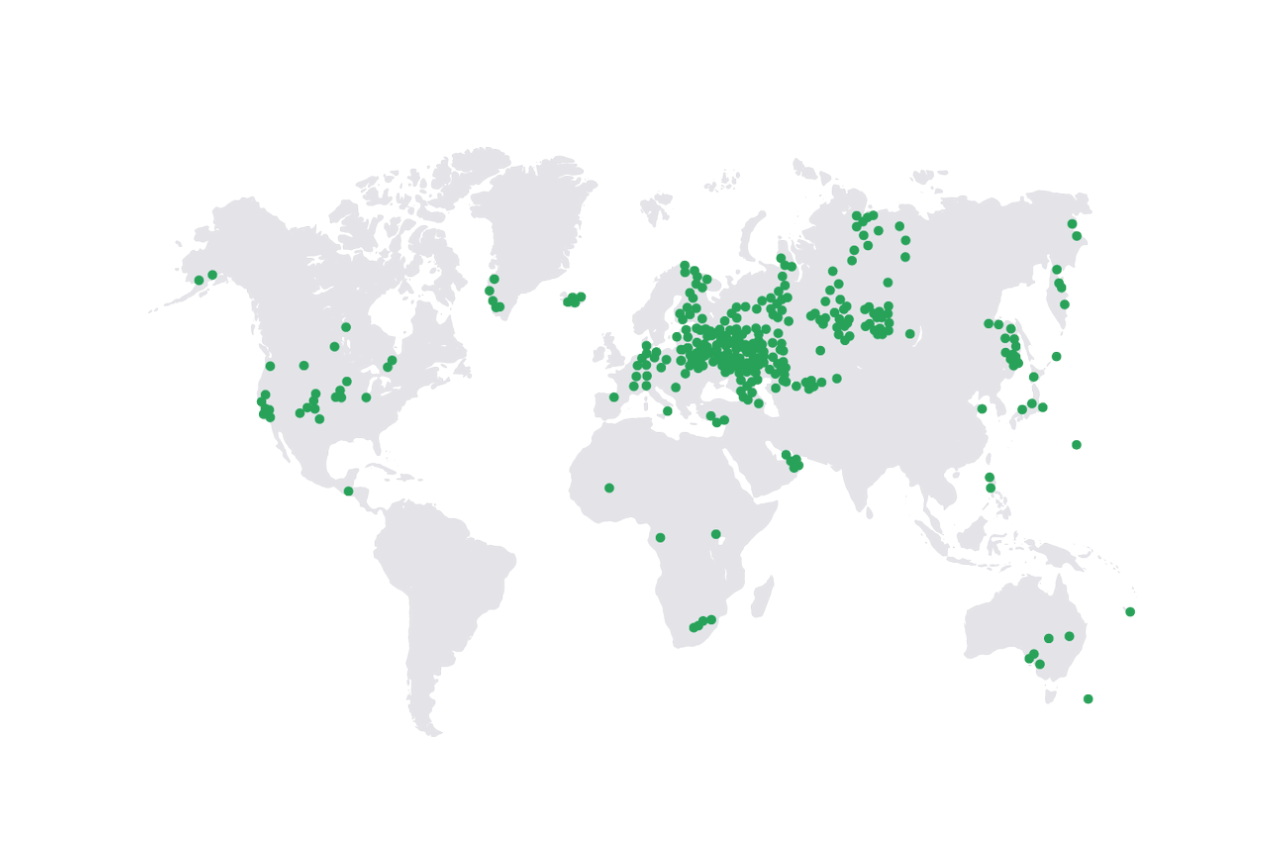
Hydrogen enthusiasts point out that hydrogen can be generated by a number of different processes beneath the earth’s surface – which is true, and explains a lot of the hydrogen shows.
But it is not enough just to generate some hydrogen. Just like natural gas, in order to achieve the cheap prices, reliability, and huge volumes claimed by Natural Hydrogen Ventures, natural hydrogen must occur in high concentrations in large volumes that can be delivered at high rates through wellbores.
In the parlance of oil and gas, we need high-volume reservoir rocks with excellent porosity (pore spaces within the rock) and permeability (capacity to flow fluids). Such rocks are almost always sedimentary – for example, sandstone or dolomite – as igneous and metamorphic rocks generally lack the well-connected pore spaces characteristic of good reservoirs. Oil and gas geologists have been studying reservoirs for more than a century, and have developed excellent models to understand their distribution.
Assuming we can find a large, high-quality reservoir to host the hydrogen, we then need one or more very impermeable rock layers above the reservoir to prevent the gas (hydrogen in this case) from escaping through fractures or just by seeping out slowly. Many rocks that are sufficiently impermeable to seal in oil or natural gas do not work for hydrogen or helium, because the molecules are very small and mobile, and can leak through just about anything. Usually a thick layer of salt or comparable material will do the trick, so that is something that helium explorers look for. So exploring for hydrogen is very much like exploring for natural gas (or helium) – we are looking for the same rocks in each case, but different gases within the rocks.
In sedimentary reservoir rocks, the gas found almost everywhere is natural gas (methane plus some heavier hydrocarbons), which is why a huge industry has been built around it. One can cover off the costs of drilling, pipelines, and other facilities by producing tens of millions of cubic feet of natural gas a day from a wellbore, fetching prices between $2 and $10 per thousand cubic feet. Helium can be produced economically in far smaller concentrations and lower volumes because it fetches a market price about 100 times that of natural gas.
But hydrogen? Industry has drilled millions of wells in sedimentary basins around the world and has found no large reservoirs full of nearly pure hydrogen. There are only traces. Where hydrogen has been detected in higher concentrations, there are no reservoirs.
Either way, there is no gold hydrogen. Green, grey, and blue hydrogen are going to have to meet the challenges of the energy transition on their own, while the pursuit of naturally occurring hydrogen should be likened to fool’s gold.